Members:
David ROUSSEAU (Imhorphen team - IRHS, University of Angers), Carole FRINDEL (Myriad Team - CREATIS, Insa de Lyon), Ali AHMAD (Myriad team- Creatis, Insa de Lyon) and Guillaume VANEL (Myriad team- CREATIS, Insa de Lyon).
Context:
Metastasis is the leading cause of cancer death among cancer patients. Cancer genomics showed that tumor progression to metastasis formation is poorly supported by further genetic alterations, implying that the adaptation capacity of disseminating tumor cells to foreign microenvironments rely on epigenetic alterations. Epigenomic reprogramming plays a central role in cancer progression and metastasis formation, supporting tumour heterogeneity, which represents a challenge for precise diagnosis and targeted therapy.

Defining the 3D-organization of cancer-associated chromatin domains would represent a new frontier to decipher tumor heterogeneity. None of the currently available technologies permit to rapidly analyze thousands of cells and profile their chromatin organization at single cell level, as needed for medical diagnosis and therapeutic guidance.
Objective:
The aim of the project is therefore to study cancer heterogeneity at single cell level by classifying freshly isolated cancer samples based on their chromatin architecture: we aim to use chromatin alteration as a marker for cancer. In order to achieve this result we will develop a superresolution microscope with high throughput capabilities, able to acquire thousands or even tens of thousands samples. Extracting and imaging large number of samples will allow performing statistically relevant biological studies that take into account the variability of cellular behaviour in the same pathological context, and creating highly specific phenotyping procedures required for precision medicine.

Ideally, microscopy devices should have a flexible design that allows customization for various samples and should be low-cost for a widespread diffusion in the context of personalized medicine. To fulfill these requirements we will develop a lattice light sheet microscope using lab-on-chip technologies: the illumination path and sample delivery systems will be fully integrated in a mm-scaled lab-on-chip. Using this device we aim to provide
high-resolution imaging of cells and sub-cellular structures as chromatin network at the diffraction limit and beyond, with minimal photo-toxicity and to generate high-throughput data by scanning them in automatic fashion within a fluidic network.
The long term vision of PROCHIP project is to develop a new enabling technology, able to extract cancer cell from a patient, assess alterations in chromatin architecture at single cell level, and define a targeted personalized therapy or drive the development of new drugs and pharmaceutical treatments.
In details the objectives of the project are to:
1) develop a miniaturized three-dimensional fluidic network integrated on a glass chip and its relative pumping system for automatic sample scanning under a microscope.
2) develop a super-resolution microscope, whose illumination and sample scanning components are integrated in a lab-on-chip (super-resolution microscope on-chip).
3) develop the protocols and the technology for isolation of primary and metastatic tumor cells suitable for high-throughput imaging and data analysis at superresolution based on the image simulator.
4) assess cancer cell heterogeneity by querying chromatin domains as functional biomarkers.
Funding and consortium:

PROCHIP project has been granted a 2.5 million from the EU H2020 FET Open programme.
The three years (2018 - 2021) project is carried out by an international consortium of 6 organizations from three European countries (Italy, France and England), led by the Italian National Reasearch Council.
PROCHIP official website: https://pro-chip.eu/
Microscopy image simulator (MicroVIP)
MicroVIP is a user friendly fluorescence microscopy image simulation and analysis tool deployed on virtual imaging platform of Creatis lab. It allows the simulation of microscopy images issued from several microscopy techniques such as Wide-Field, Confocal, Structured Illumination Microscopy (SIM) and Light Sheet Microscopy (LSM). 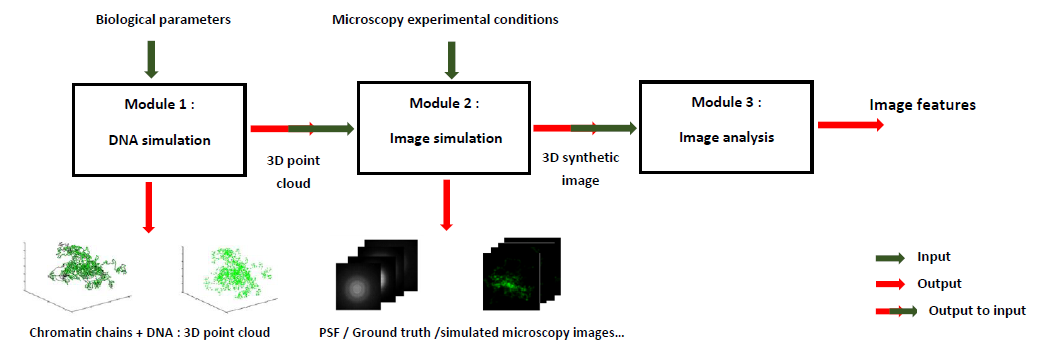
Microscopy image simulation allows one to define and virtually perform microscopy experiments of modelled cells. It can be used to assess the effect of several parameters on the output microscopy images before conducting a real experiment. Moreover, this lets one generate a large number of call images for an extremely low cost, making it a valuable tool for training or testing image analysis algorithms and validating their behavior when faced with several experimental conditions (sub-resolved or super-resolved images, variable cell phenotypes...). Many single cell microscopy simulators have been proposed, but they usually are specific to a single microscopy technique. They also lack standardisation, and thus do not share common sets of inputs or outputs, common programmation languages, platforms, or types of interface. It is therfore a challenge for one to find the software they need with respect to their expertise, hardware constraints, experimental conditions, etc.
With this in mind, we present MicroVIP, a single user-friendly application gathering simulators for a wide range of microscopy technique. In addition to offering a very easy-to-understand graphical interface, MicroVIP comes with comprehensive parameters customization capabilities for flexibility and adaptation to the experimenter needs. Notably, it even allows simulation of the imaging process of cells in motion in a microfluidic system, with custom cell speed. Furthermore, MicroVIP implements a complete and self-sufficient simulation pipeline raher than only modeling the image acquisition process. It indeed integrates a module of generation of the ground truth cell chromatin chains and fluorescent markers positions, as well as another one performing commonly used features extraction methods after the simulated image has been obtained. Lastly, its deployment on Creatis' Virtual Imaging Platform (VIP) allows the user to run the software without concerns about installation procedures, dependencies of hardware constraints.

Microscopy image simulator (MicroVIP): avaible on VIP
MicroVIP demo (to be updated soon): here
MicroVIP source code: Coming Soon
Related publications:
AHMAD, Ali, FRINDEL, Carole, et ROUSSEAU, David. Detecting differences of fluorescent markers distribution in single cell microscopy: textural or pointillist feature space?. Frontiers in Robotics and AI, 2020, vol. 7, p. 39.
A. Ahmad, C. Frindel and D. Rousseau, "Sorting cells from fluorescent markers organization in confocal microscopy: 3D versus 2D images," 2020 Tenth International Conference on Image Processing Theory, Tools and Applications (IPTA), Paris, 2020, pp. 1-6, doi: 10.1109/IPTA50016.2020.9286463.
ALI, Ahmad, FRINDEL, Carole, et ROUSSEAU, David. Détection de différence de densité de marqueurs fluorescents en microscopie superrésolue: approche pointilliste ou texturale?. In : XXVIIe colloque Gretsi. 2019.
AHMAD, Ali, RASTI, Pejman, FRINDEL, Carole, et al. Deep learning based detection of cells in 3D light sheet fluorescence microscopy. In : Quantitative BioImaging Conference (QBI 2019). 2019.
T. Glatard, C. Lartizien, B. Gibaud, R. Ferreira da Silva, G. Forestier, F. Cervenansky, M. Alessandrini, H. Benoit-Cattin, O. Bernard, S. Camarasu-Pop, N. Cerezo, P. Clarysse, A. Gaignard, P. Hugonnard, H. Liebgott, S. Marache, A. Marion, J. Montagnat, J. Tabary, and D. Friboulet, A Virtual Imaging Platform for multi-modality medical image simulation, IEEE Transactions on Medical Imaging, vol. 32, no. 1, pp. 110-118, 2013



