Coordinators | ||
CliniciansJean-Christophe RICHARD <j-christophe.richard at chu-lyon.fr> | Laurent BITKER <laurent.bitker at chu-lyon.fr> | |
Image ProcessingMaciej ORKISZ <maciej.orkisz at creatis.insa-lyon.fr> | Emmanuel ROUX <emmanuel.roux at creatis.insa-lyon.fr> |
About
To propose and validate image-assisted personalized ventilation strategies and, ultimately, reduce patient mortality, our group is conducting both medical and image-processing research.
Images, collected within preclinical and clinical studies and carefully annotated, build up databases used to assess biomarkers such as alveolar recruitment and hyperinflation, but also to train automatic image segmentation and alignment algorithms. The latter, implemented in prototype software are, in turn, used and evaluated within subsequent trials.
Clinical and pre-clinical studies
Patients
- CT4ARDS, clinicalTrials NCT03870009; completed.
- 31 patients (11 COVID+), 2 CT scans/patient.
- VT4COVID, clinicalTrials NCT04349618; completed.
- 215 patients.
- CT4ARDS-2, clinicalTrials NCT06113276; recruiting.
- >160 patients (almost all COVID+), 4 CT scans/patient.
Porcine experimental model
- PK TCM, 2021, development of a specific quantification method by multi-compartment pharmacokinetic modeling of [11C](R)-PK11195 pulmonary uptake in pigs with or without experimental ARDS; completed.
- 21 pigs, PET imaging.
- PK DV, 2023, evaluation of the impact of prone position on macrophagic lung inflammation quantified by pulmonary uptake of [11C](R)-PK11195 during experimental ARDS; completed.
- 11 pigs, PET-CT imaging.
- PK HIDRA, 2023, evaluation of the impact of a very low tidal volume ventilation strategy under veno-venous ECMO on macrophagic lung inflammation quantified by pulmonary uptake of [11C](R)-PK11195 during experimental ARDS; completed.
- 10 pigs, PET-CT imaging.
Selected results
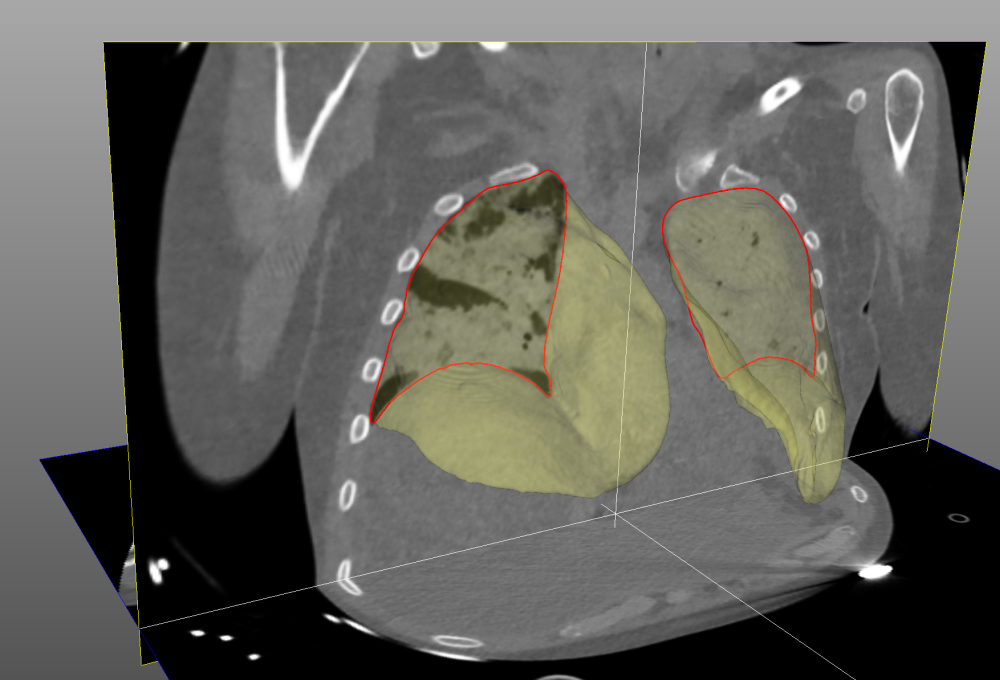
Prototype software [Dávila Serrano et al. ICCVG 2020] with:
- an interactive module considerably reducing the manual segmentation time, used for reference measurements and annotations,
- a semi-automatic module segmenting the aerated regions of the lungs, used to assess cyclic hyperinflation and adjust the tidal volume of the ventilator,
- a fully automatic module implementing an artificial intelligence method for lung segmentation.
Automatic lung segmentation method
The best results have been recently obtained using an artificial neural network called 3D U-net. The algorithm, trained on 316 annotated 3D CT scans from 97 patients and evaluated on 118 scans from 34 patients with moderate-to-severe ARDS, achieved high overlap (96% Dice score) and low average surface distance (1.2mm ASSD) compared with reference annotations [Peñarrubia, PhD thesis 2022]. Its uncertainties are on the order of inter-observer variability [Peñarrubia, et al. ICMX 2023] and clinicians estimate that 85% of the automatically segmented lung masks are exploitable without corrections.
Clinical
In patients with moderate-to-severe COVID-19-related ARDS alive at day 60, no significant difference was observed between those ventilated with ultra-low and low tidal volume, which does not argue in favor of the routine use of ultra-low tidal volume in patients with COVID-19-related ARDS [Richard J.-C., et al. The Lancet, Resp Med 2023].
Lung recruitability of COVID-19 pneumonia was not significantly different between ECMO and non-ECMO patients, with substantial interindividual variations. The balance between hyperinflation and recruitment induced by PEEP increase from 5 to 15 cmH2O appeared favorable in virtually all ECMO patients. Compliance of the aerated lung was significantly lower in ECMO than in non-ECMO patients, independently of lung recruitability [Richard J.-C., et al. Crit Care 2022].
COVID-related ARDS patients share similar CT features with non-COVID patients (increased lung weight, increased noninflated lung fraction). A subtype of COVID-19 ARDS patients with near-normal elastance presented with low tidal recruitment, low amount of non-inflated lung, and high amount of normally aerated lung, questioning the relevance of high PEEP levels in this subgroup [Chauvelot L., et al. J Crit Care 2020].
Experimental
In an experimental model of ARDS, 4h of prone positioning significantly decreased acute lung inflammation in all lung regions, consecutive te decreased biomechanical forces and changes in the respiratory system mechanics [Dhelft F., et al. J Appl Physiol 2023].
A three-tissue compartment kinetic model significantly improved the assessment of [11C](R)-PK11195 lung uptake in most lung regions. This new methodology offers the opportunity to non-invasively evaluate innovative ventilatory strategies aiming at controlling acute lung inflammation [Bitker L., et al. EJNMMI 2022].
A novel multiple-breaths nitrogen washin–washout technique that reliably assesses EELV at bedside, and might add valuable information to further personalization of mechanical ventilation [Bitker L., et al. ICMX 2021].
Funded projects
IAVAI (Image-Assisted Ventilation using Artificial Intelligence)
- Maturation project aiming to guarantee high accuracy and robustness of our lung segmentation method, 2024-25.
- Funded by the COMS@N consortium within the programme France 2030.
PK VIKI
- Evaluation of the impact of high tidal volume ventilation on renal perfusion, oxygenation and inflammation in a porcine model using functional PET-MRI. 12 pigs, ongoing.
- Funded by SRLF (Société de réanimation de langue française, 2023) and HCL Foundation (Hospices Civils de Lyon, 2023).
PK APNEA
- Impact of apneic ventilation under ECMO on pulmonary inflammation.
- Funded by HCL (Hospices Civils de Lyon) as part of JCJC (young researchers) programme, 2023.
Selected publications
Journals
Richard J.-C., et al. “Ultra-low tidal volume ventilation for COVID-19-related ARDS in France (VT4COVID): a multicentre, open-label, parallel-group, randomised trial”, The Lancet, Respiratory Medicine, 11:11, 991-1002, 2023, DOI: 10.1016/S2213-2600(23)00221-7.
Dhelft F., et al., “Prone position decreases acute lung inflammation measured by [11C](R)-PK11195 positron emission tomography in experimental acute respiratory distress syndrome”, Journal of Applied Physiology, 134:2, 467-481, 2023, DOI: 10.1152/japplphysiol.00234.2022.
Penarrubia L., et al., “Precision of CT-derived alveolar recruitment assessed by human observers and a machine learning algorithm in moderate and severe ARDS”, Intensive Care Medicine Experimental, 2023, 11, 8. DOI: 10.1186/s40635-023-00495-6.
Richard J.-C., et al. “Response to PEEP in COVID-19 ARDS patients with and without extracorporeal membrane oxygenation. A multicenter case-control computed tomography study”, Critical Care, 26, 195, 2022, DOI: 10.1186/s13054-022-04076-z.
Bitker L., et al. “Non-invasive quantification of acute macrophagic lung inflammation with [11C](R)-PK11195 using a three-tissue tissue compartment kinetic model in experimental acute respiratory distress syndrome”, European Journal of Nuclear Medicine and Molecular Imaging, 49, 2122–2136, 2022, DOI: 10.1007/s00259-022-05713-z.
Bitker L., et al. “Validation of a novel system to assess end-expiratory lung volume and alveolar recruitment in an ARDS model”, Intensive Care Medicine Experimental, 9, 46, 2021, DOI: 10.1186/s40635-021-00410-x.
Chauvelot L., et al. “Quantitative-analysis of computed tomography in COVID-19 and non COVID-19 ARDS patients: a case-control study”, Journal of Critical Care, 60, 169-176, 2020, DOI : 10.1016/j.jcrc.2020.08.006.
Penarrubia L., “Quantification de l’aération pulmonaire sur des images CT de patients atteints du syndrome de détresse respiratoire aiguë”, PhD thesis 2022LYO10164, EDISS, Univ. Lyon 1, Dec. 2022.
Conferences
M. Shekarnabi M., et al. “CT registration-derived biomarkers of recruitability in ARDS”, IEEE International Symposium on Biomedical Imaging, Cartagena de Indias, Colombia, 2023, DOI: 10.1109/ISBI53787.2023.10230395.
M. Shekarnabi M., et al. “Phenotypes of functional CT imagining predict clinical outcome in ARDS patients”, European Respiratory Society (ERS) International Congress, Milan, Italy, 2023, DOI: 10.1183/13993003.congress-2023.OA4955.
Dávila Serrano E. E., et al. “Software for CT-image Analysis to Assist the Choice of Mechanical-ventilation Settings in Acute Respiratory Distress Syndrome”, International Conference on Computer Vision and Graphics, Warsaw, Poland, 2020, Springer, LNCS 12334, pp 48-58, DOI: 10.1007/978-3-030-59006-2_5.